How Is an Enzyme Like a Lock and Key
Only the correctly sized and shaped substrates can bind with the enzyme in. The binding of substrate to the enzyme can occur in two mechanisms.

Difference Between Lock And Key Hypothesis And Induced Fit Hypothesis Choice Questions Biology Notes Lock And Key
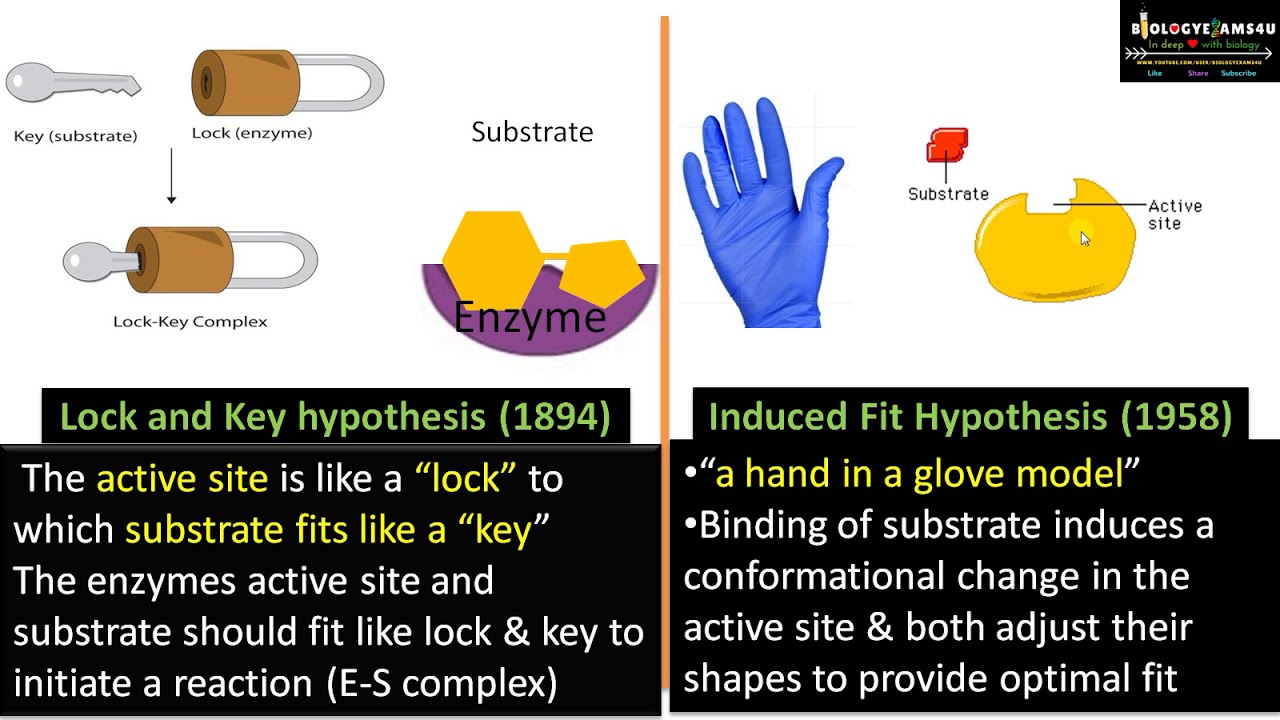
Lock and Key Hypothesis.
. However a competitive inhibition is usually reversible if sufficient. Even if the substrate is the correct substrate for an enzyme an allosteric inhibitor can prevent the enzyme from having the correct shape or conformation. In this model an enzymes active site is a specific shape and only the substrate will fit into it like a lock and key.
The lock and key theory utilizes the concept of an active site The concept holds that one particular portion of the enzyme surface has a strong affinity for the substrate The substrate is held in such. In other words an enzyme that catalyzes one reaction wont have any effect on a different reaction. The substrate does not covalently bind to the active site but weakly interacts with it through interactions like hydrogen-bonding van der Waals interactions etc.
The inhibitor competes for the same active site as the substrate molecule. The interactions between the substrate and active site are weak noncovalent interactions ie. Both models depend on the degree of precise binding of the substrate to the active site of the enzyme.
If the substrate cannot fit into an active site the enzyme cannot catalyze a reaction. The Þrst the lock-and-key model assumes a high degree of similarity between the shape of the substrate and the geometry of the binding site on the enzyme Figure 63a. In many cases however the configurations of both the enzyme and substrate are modified by substrate bindinga process called induced fit.
They are important in describing how enzymes increase the rate of a biological reaction through. Induced fit and lock and key are the two models which describe the mechanism of action of the enzyme. Enzymes do generally work in a lock and key fashion in terms of the enzyme representing a lock and the substrate representing the key The substrate fits an enzymes active site similar to how.
But there are two important theories that we will discuss here. The co-factors are usually vitamins consumed through various food sources and open up the active site on the enzyme. According to a passage about metabolism published by Titan Education this is not entirely accurate because some enzymes break up unevenly at the end of the catalytic process.
A theory called the lock-key theory of enzyme catalysts can be used to explain why inhibition occurs. Only one key can open a lock correctly. Like other catalysts enzymes change the equilibrium of a reaction but they arent consumed in the process.
The wrong key will not fit the specific lock on an enzyme. In 1894 scientist Emil Fischer called this model the lock-and-key model because the enzyme and substrate fit together like a key in a lock. ELISA ELISA - an acronym for Enzyme-Linked ImmunoSorbent Assay.
There are many theories that explain how enzymes work. Allosteric Inhibition Inhibits Enzymatic. The inhibitor is stuck on the enzyme and prevents any substrate molecules from reacting with the enzyme.
The ELISA assay uses the coupling of antigens and antibodies and relies on the specificity and affinity of antibodies for antigens. In such cases the conformation of the substrate is. 1Lock-and-Key Mechanism Gale.
The binding slightly changes the structure of the enzyme. The lock and key model was first proposed in 1894. The lock-and-key model states that the substrate acts as a key to the lock of the active site.
The active site and substrate are exact matches for each other similar to. The inhibitor may interact with the enzyme at the active site but no reaction takes place. The lock-and-key model asserts that substrate fits exactly with the enzyme in one instantaneous step.
The substrate binds to a site whose shape complements its own like a key in a lock or the correct piece in a three-dimensional jigsaw puzzle. This enzyme-substrate molecule now reacts with the second substrate to form the product and the enzyme is liberated as the second product. A good way to think about this is a lock-and-key model.
Active sites are where reactions take place on an enzyme and can only act upon one substrate which can be other proteins or sugars. While most catalysts can act on a number of different types of reactions a key feature of an enzyme is that it is specific. This is the difference between induced fit and lock and key.
This model has intuitive appeal but is now largely. Lock-and-key model and induced fit model. Similar to a lock and key substrate and enzyme fit with each other very tightly according to this hypothesis.
Similarities Between Induced Fit and Lock and Key Model. The enzyme and substrate interact to form an enzyme-substrate complex. The simplest model of enzyme-substrate interaction is the lock-and-key model in which the substrate fits precisely into the active site Figure 224.
In the induced fit theory the active site of the enzyme is not static while it is static in the lock and key mechanism. The ELISA assay is a widely used biochemical assay to detect in a sample the presence of and quantity of proteins such as hormones and antibodies and bacteria or viruses.

Difference Between Lock And Key Hypothesis And Induced Fit Hypothesis Choice Questions Biology Notes Lock And Key

Lock And Key Model A Model For Enzyme Action Enzymes Biology Enzymes What Is A Product

Gcse Biology Enzymes Youtube An Overview Of How Enzymes Work Focusing On The Lock And Key Diagram If You Have Any Questio Enzymes Biology Enzymes Biology

No comments for "How Is an Enzyme Like a Lock and Key"
Post a Comment